Dr. Hany Aziz

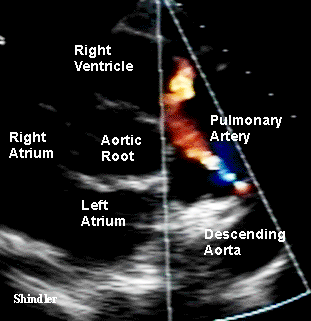
Signs and symptoms are proportionate to size of the shunt
from aorta to pulmonary artery.
A small ductal shunt will prompt referral for echocardiography
because of a murmur.
The murmur is usually detected in the first few weeks of life.
It may only be systolic rather than the typical continuous
murmur.
The typical murmur increases in intensity through systole
becoming loudest at the time of aortic valve closure. It
continues without interruption into diastole, decreasing
in intensity but assuming a higher frequency.
There may be "shaking dice" noises, or clicks in systole.
Increased flow through the mitral valve may result in an
apical diastolic rumble.
The ductus murmur can be distinguished from a venous hum
by the location where it is heard loudest and by turning
the head during auscultation. The hum disappears when the head
is turned. The ductal murmur is loudest at the second left
intercostal space. The venous hum may be loudest at the
supraclavicular fossa, or on the right side of the sternum.
Venous hums are also modified by respiration and by a change
in body position.
Other causes of continuous murmurs include:
ruptured sinus of Valsalva aneurysm,
coronary artery fistula, aortopulmonary window, collateral flow
murmurs in tetralogy with pulmonary atresia.
Tetralogy of Fallot with pulmonary atresia should always be
suspected in a cyanotic neonate with continuous murmurs heard
over the back.
Fripp RR, Whitman V, Waldhausen JA, Boal DK. Ductus arteriosus aneurysm presenting as pulmonary artery obstruction: diagnosis and management. J Am Coll Cardiol 1985 Jul;6(1):234-6
The occurrence of pulmonary artery obstruction in an 8 day old infant as a complication of an aneurysm of a nonpatent ductus arteriosus is reported, together with the echocardiographic and angiographic findings. To relieve the obstruction, the aneurysm and an intrapulmonary thrombus were successfully removed with the use of cardiopulmonary bypass when the infant was 3 months old.
Catheter Cardiovasc Interv. 2005 Jan;64(1):91-101.
Interventional treatment of patent ductus arteriosus in 2004.
Moore JW, Levi DS, Moore SD, Schneider DJ, Berdjis F.
Division of Pediatric Cardiology, Mattel Children's Hospital, University of California, Los Angeles, California.
In 2004, the interventional treatment of patent ductus arteriosus (PDA) is definitive and curative. In current practice, coils are used for smaller PDA, and devices are employed for larger PDA. Developing technologies offer small improvements in control a nd results, but do not appear to promise major changes in practice. This review summarizes the current and emerging interventional technologies directed at PDA closures.
Indian Heart J. 2004 May-Jun;56(3):232-4.
Transcatheter management of patent ductus arteriosus in sick ventilated small infants.
Kannan BR, Anil SR, Padhi SS, Kumar RK.
Department of Cardiology, Amrita Institute of Medical Sciences and Research Centre, Kochi.
BACKGROUND: Large patent ductus arteriosus can present in infancy with congestive cardiac failure and superadded pulmonary infection can necessitate mechanical ventilation. Surgical intervention is traditionally indicated for this subset of patients. We p resent our experience of transcatheter coil closure of the patent ductus arteriosus in such infants. METHODS AND RESULTS: Five infants weighing between 960 gm and 4.1 kg, aged between 17 days and 3 1/2 months were mechanically ventilated because of conges tive cardiac failure with pneumonia. Echocardiography showed patient ductus arteriosus with a size of 1.8 to 4.2 mm and adequate ampulla. Bioptome-assisted coil delivery was done and successful patient ductus arteriosus closure was achieved in all. There were two instances of embolization of coils with successful retrieval and redeployment. All infants could be weaned off mechanical ventilation over the next 24-72 hours. A pre-term infant developed a Doppler gradient of 25 mmHg in the descending aorta tha t decreased to 12 mmHg five months later. There was no significant obstruction to pulmonary artery flow in any child. At three months follow-up, all the five infants were asymptomatic with no residual flow across the patient ductus arteriosus. CONCLUSIONS : Transcatheter coil closure of moderate to large patent ductus arteriosus is possible in sick ventilated infants weighing below 5 kg. It may be a better alternative to surgery in selected cases in view of minimal morbidity.
Pulmonary and aortic valve endocarditis in an adult patient with silent patent ductus arteriosus.
Ozkokeli M, Ates M, Uslu N, Akcar M.
Department of Cardiovascular Surgery, Abant Izzet Baysal University, Izzet Baysal Medical Faculty.
Pulmonary and aortic valve endocarditis are uncommon especially in an adult patient with patent ductus arteriosus. A 27-year-old woman diagnosed with pulmonary and aortic valve endocarditis underwent surgical treatment. Here, we report our clinical and su
rgical experience in treating a case of double valve endocarditis with clinically silent patent ductus arteriosus.
Vnitr Lek. 2004 Nov;50(11):873-6.
Syncope in ventricular tachycardia as a first clinical sign of patent ductus arteriosus in an adulthood
Praus R, Parizek P, Cervinka P, Haman L, Tauchman M, St'asek J, Pudil R, Gregor J, Hudik M, Hodac M.
II. interni klinika Lekafske fakulty UK a FN, Hradec Kralove.
The case-report describes a 48-year-old-female patient with patent ductus arteriosus with the following structural changes leading to the malignant arrhythmias manifested as a syncope. The patient was treated by Amplatzer occluder and implantation of a ca
rdioverter-defibrillator. The authors discuss patent ductus arteriosus, arrhythmias and sudden cardiac death in adult patients with congenital heart disease.
Arch Med Res. 2004 Nov-Dec;35(6):549-53.
Risk factors for the development of bronchopulmonary dysplasia: a case-control study.
Hernandez-Ronquillo L, Tellez-Zenteno JF, Weder-Cisneros N, Salinas-Ramirez V, Zapata-Pallagi JA, da Silva O.
BACKGROUND: Advances in neonatal care over the past decades have meant that an increasing number of very premature infants survive today than in years past. One of the main factors contributing to the survival of these infants is development in ventilator
y support. However, this has led to lung injury and an increase in the incidence of bronchopulmonary dysplasia (BDP). METHODS: A case-control study was conducted at the National Institute of Perinatology Neonatal Intensive Care Unit in Mexico City, Mexico
to evaluate the risk factors associated with the development of BPD in premature infants requiring ventilatory support within the first days of life for respiratory failure. Twenty two cases and 22 control premature infants admitted to the Neonatal Unit
requiring assisted ventilation and that survived for more than 28 days were included. The neonatal and maternal risk factors that were considered for analysis were the following; mode of delivery, antenatal steroids, gestational age, birth weight, Apgar s
cores, sepsis, patent ductus arteriosus, and ventilation parameters. RESULTS: Factors associated with the development of BPD were late sepsis (OR 7.29, 95% CI 1.61-35.8, p=0.002), and two or more episodes of sepsis (OR 7.60, 95% CI 1.46-44.6, p=0.004). Ot
her risk factors were low birth weight and younger gestational age at birth. CONCLUSIONS: Similar to what has been reported by other investigators in developed countries, our study showed that neonatal sepsis, low birth weight, and gestational age were as
sociated with BPD in our patients.
Echocardiography. 2004 Oct;21(7):665-7.
Patent ductus arteriosus complicated by pulmonary artery endarteritis in an adult.
Kadakia R, Giullian J, Dokainish H.
Department of Medicine, Section of Cardiology, Baylor College of Medicine, Houston, Texas 77030, USA.
Am J Forensic Med Pathol. 2004 Sep;25(3):200-4.
Patent ductus arteriosus as a natural cause of pulmonary hemorrhage in infants: a medicolegal dilemma.
Lewis MJ, McKeever PK, Rutty GN.
Division of Forensic Pathology, University of Leicester, Leicester Royal Infirmary, Leicester, UK.
Patent ductus arteriosus (PDA) is a recognized risk factor for massive pulmonary hemorrhage (MPH) in the newborn and is generally seen in association with other MPH risk factors such as prematurity. We report 6 cases of sudden and unexpected death of infa nts older than 4 days with MPH and PDA at autopsy. The cases were reviewed for other factors that could contribute to MPH to ascertain whether PDA is directly linked to MPH. Histology samples were examined for distribution of hemorrhage in the lungs and i ron stained for hemosiderin evaluation. All of the cases had clinical histories and scene examinations which raised the differential diagnosis of mechanical asphyxia in the form of so-called overlayings. The diagnostic dilemma of attributing the MPH to th e PDA as the sole cause, dual cause, or incidental finding is discussed. These cases illustrate the medicolegal dilemma faced by the pathologist, as well as the need for further research into the potential association of PDA with MPH.
Arq Bras Cardiol. 2002 Sep;79(3):302-7. Epub 2002 Oct 08.
Silent patent ductus arteriosus aneurysm.
Botta AM, Aquino F, Pereira C, Fin A, Nogueira A, Ricachinewsky C, Lucchese F, Molossi S.
Irmandade da Santa Casa de Misericordia de Porto Alegre, Brazil. abotta@terra.com.br
Ductus arteriosus aneurysm, a rare and potentially fatal condition, has been reported as a complication after surgical ductus arteriosus closure. Its spontaneous appearance as a septic complication, which was common in the presurgical and preantibiotic er a, has been rarely reported in the contemporary literature. Persistence of silent ductus arteriosus in healthy children and adults is a frequent condition that currently has an increasing diagnostic possibility due to the availability of more accurate inv estigative methods, especially echocardiography. We report the case of a 1-year-old child, in whom no previous heart disease was known, who developed a giant aneurysm of the ductus arteriosus during a staphylococcal infection. This complication appeared a fter craniotomy for emptying an accidental subdural hematoma. This report associates the persistence of ductus arteriosus with a complication considered rare, which has a rapidly fatal evolution.
Pediatr Cardiol. 2003 Jan-Feb;24(1):27-30.
Silent and audible persistent ductus arteriosus: an angiographic study.
Bennhagen RG, Benson LN.
Department of Pediatrics, Lund University Hospital, 221 85 Lund, Sweden.
Persistent ductus arteriosus (PDA) murmurs become silent probably due to the direction of the jet across the ductus arteriosus when entering the pulmonary artery. Out of 15 children with silent PDA, 14 demonstrated a ductal flow not contacting and away fr om the anterior wall of the main pulmonary artery. In 15 children with a continuous murmur caused by a PDA, 12 exhibited a ductal flow toward and reaching the anterior wall of the MPA. There was no correlation between the presence of a murmur and the size of the arterial duct in this study.
Curr Interv Cardiol Rep. 2001 Aug;3(3):268-274.
Summary and Comparison of Patent Ductus Arteriosus Closure Devices.
Rao PS.
Division of Pediatric Cardiology, Saint Louis University School of Medicine, Cardinal Glennon Children's Hospital, 1465 South Grand Boulevard, St. Louis, MO 63104, USA. raops@slu.edu
A number of patent ductus arteriosus (PDA) occluding devices have been studied in an attempt to develop a transcatheter method of closure of PDA. Some devices were tested in only animal models, whereas others progressed to clinical trials in human subject s. Some devices have been discontinued, some received approval for general clinical use, and many have not yet received approval by regulatory authorities. No prospective randomized clinical trials have been undertaken and, therefore, data on separate cli nical trials are used to determine relative efficacy of the devices. Selection of a method of PDA closure depends largely on its minimal diameter and to some degree on its shape. Silent PDAs do not need occlusion. Very small to small PDAs may be occluded by free or detachable Gianturco coils (Cook Cardiology, Bloomington, IN). Moderate-to-large PDAs require closure by devices, conventional surgery, and videothoracoscopic interruption. The choice in the latter group depends largely on the availability of a given method at a given institution at that particular time. Approval of several of the devices by regulatory authorities may result in conduct of prospective randomized clinical trials and use of a device or method most appropriate to the size and shape of the PDA.
Heart. 2000 Dec;84(6):619.
Images in cardiology: silent patent ductus arteriosus endarteritis.
Parthenakis FI, Kanakaraki MK, Vardas PE.
Am Heart J. 1996 Oct;132(4):905-9.
Comment on:
Am Heart J. 1996 Oct;132(4):827-35.
Transcatheter occlusion of patent ductus arteriosus: which method to use and which ductus to close.
Rao PS.
Many devices have been developed for transcatheter occlusion of PDA. Bulkiness of the device, complexity of the procedure, and significant residual complications make the majority of the devices unsuitable for routine clinical use. Although no randomized comparative clinical trials exist, on the basis of published literature and my personal experience, coil occlusion may be best suited to close small ducts (< or = 3.5 mm) and the adjustable buttoned device may be most appropriate for large PDAs (> 3.5 mm) . Clinical trials on larger patient populations than are currently available and long-term follow-up are necessary to further support these recommendations. Indications for transcatheter closure should be exactly as those used for surgical closure: PDA wi th audible continuous murmur with echo Doppler confirmation. The so-called silent ducts need not be closed.
J Perinat Med. 1995;23(6):493-9.
"Silent" patent ductus arteriosus and bronchopulmonary dysplasia in low birth weight infants.
Zanardo V, Trevisanuto D, Dani C, Milanesi O, Secchieri S, Guglielmi A, Brentegani L, Cantarutti F.
Department of Pediatrics, Padua University, Italy.
We conducted a clinical study on the antecedents of bronchopulmonary dysplasia (BPD) in 290 premature RDS infants with < or = 1.75 kg birth weight (BW). They were enrolled in a prospective trial of indomethacin treatment for "silent" patent ductus arterio sus (PDA), screened by 2-D echocardiographic and pulsed Doppler evaluation on the third day of life. The trial took place at the NICU of the Pediatric Department of Padua University between January 1987 and December 1991. Out of 290 infants screened, 96 h ad evidence of "silent" PDA (33%) and 77 responded to indomethacin treatment (80%). Comprehensively 79 (27%) developed BPD, and from these the incidence of BPD was statistically increased in infants with "silent" PDA, 47 out of 96 (49 +/- 9%), with respec t to 32 out of 194 (16 +/- 3%) preterm infants without PDA. Statistical analysis showed that in preterm infants with "silent" PDA the development of BPD was correlated at 99% C.L. to their low BWs (mean BW = 1.13 kg): in fact the mean and the mode of BW d istributions were statistically lower in the presence of BPD, 1.03 kg versus 1.24 kg, and 0.88 kg versus 1.65 kg respectively. Moreover, the preterm infants with "silent" PDA unresponsive to the first course of indomethacin and/or submitted later to surgi cal closure, presented a statistically lower BW with respect to the early responders, 1.06 kg versus 1.18 kg, and at the same time a statistically higher incidence of BPD (63 +/- 20% versus 43 +/- 9%). From these data we conclude that, although "silent", PDA increase per se the incidence of BPD, even if benefits from an early induced closure. Furthermore, a lower BW of infants affected by "silent" PDA represents a contributing factor to the development of BPD.
Klin Padiatr. 1993 Sep-Oct;205(5):354-6.
Silent persistent ductus arteriosus
Schumann D, Schickendantz S, Borowski A, Mennicken U.
Kinderkardiologie der Klinik und Poliklinik fur Kinderheilkunde, Universitat zu Koln.
We report on 14 observations undertaken by ourselves of silent persistent ductus arteriosus (SPDA). During a three year period with the routine use of color doppler flow evaluation we found cases of SPDA in 14 children. A very little ductus arteriosus is already known to us through earlier heart catheter examinations which were undertaken because of other isolated heart defects (16 cases in 345 isolated heart defects over 10 years). We also consider the fact that SPDA is not an infrequent result after the occlusion of a patent ductus arteriosus with the Rashkind-Occluder-System and after operative ligatures of patent ductus arteriosus. Finally, we compare our results concerning the frequency of SPDA with current literature and discuss consequential therap ies.
Br Heart J. 1991 Feb;65(2):97-9.
Comment in:
Br Heart J. 1993 Feb;69(2):193.
Br Heart J. 1993 Feb;69(2):193.
Doppler ultrasound and the silent ductus arteriosus.
Houston AB, Gnanapragasam JP, Lim MK, Doig WB, Coleman EN.
Department of Cardiology, Royal Hospital for Sick Children, Glasgow.
A clinically undetectable, small ductus arteriosus was identified by Doppler ultrasonography in 21 individuals. Infants were excluded from the study and no patient had pulmonary hypertension. Persistence of the ductus arteriosus is likely to be more commo n than shown by less sensitive diagnostic methods. Some patients considered to have infective endocarditis with a normal heart may have a silent ductus arteriosus. Evidence of such an association would justify ligation or antibiotic cover as prophylactic measures.
J Perinat Med. 1991;19(4):291-5.
Early screening and treatment of "silent" patent ductus arteriosus in prematures with RDS.
Zanardo V, Milanesi O, Trevisanuto D, Rizzo M, Ronconi M, Stellin G, Cantarutti F.
Department of Pediatrics, University of Padova, Italy.
From January 1987 to December 1989, prematures with RDS weighing 1750 g or less admitted to the Neonatal Intensive Care Unite (NICU) were submitted from the third postnatal day to serial two-dimensional and pulsed Doppler (ATL MK 600) echocardiographic ev
aluation for "silent" patent ductus arteriosus (PDA). PDA was diagnosed in 36/175 prematures with RDS (20.5%). Thirty patients had indomethacin treatment and the PDA closed completely in 27 (90%); five needed a second course of indomethacin, that was effe
ctive in two (40%). Four RDS patients (4/36-11%) already weaned from the respirator, needed supplemental oxygen. The three non-responders and six other prematures with counterindications to the drug underwent surgical ligation (25%). As historical control
, we retrospectively evaluated the population of preterm infants with RDS weighing less than or equal to 1750 g treated for hemodynamically significant PDA during three previous years before the screening protocol; in this group the echocardiographic and
Doppler evaluations were done when congestive heart failure and pulmonary edema were clinically evident. In comparison, we found a reduced incidence of prematures with RDS treated for PDA, 7/120 (5.8%) a higher age at medical treatment (9 +/- 1.4 vs 4.4 -
2.3 days) and a larger prevalence of patients underwent ductal ligation (71.4%). These data show that early screening and treatment of "silent" PDA may result advantageous to improve the efficacy (90%) of indomethacin, in a critical time for the recovery
of RDS, and furthermore decreasing the need of surgical ligation.
Rev Esp Cardiol. 1990 Jun-Jul;43(6):410-2.
Silent uncomplicated patent ductus arteriosus in children. Diagnosis with echo-Doppler
Salazar J, Olivan P, Ibarra F, Gutierrez A, Felipe J, Garcia MD, Lasarte JJ.
Servicio de Cardiologia Pediatrica, Hospital Miguel Servet, Zaragoza.
Three cases are presented of patent uncomplicated ductus arteriosus in children, with short systolic murmur, in which the noninvasive diagnosis could not be possible without the echo-Doppler help. Although are cases with small shunts and hemodynamically w ell tolerated, its diagnosis and surgical treatment are essential in order to prevent the risk of bacterial endocarditis of those patients.
Pediatr Cardiol. 1986;7(3):121-7.
The silent ductus: its precursors and its aftermath.
Hammerman C, Strates E, Valaitis S.
Prophylactic closure of the patent ductus arteriosus has been recommended as a means of decreasing the morbidity of the very low birth weight neonate. This study was undertaken in order to determine potential risk factors involved in the development of th e silent ductus, its impact upon both the early cardiorespiratory symptomatology and the subsequent morbidity of the premature neonate, and finally the potential benefit to be derived from prophylactic closure in this presymptomatic stage. Infants with bi rth weights of 1000 g or less were studied on days 2-3 of life echocardiographically, clinically, and with determination of plasma dilator prostaglandin levels. On entry to the study, those infants with early evidence of silent left-to-right patent ductus arteriosus (PDA) shunting were randomized to receive either prophylactic indomethacin or placebo therapy. Those infants with no evidence of ductal shunting were not treated at all. Infants with silent PDAs had elevated levels of the dilator prostaglandin metabolite 6-keto PGF1 alpha on admission, although they had no echocardiographic abnormalities. No other risk factors for PDA development could be identified. Silent PDA infants had an increased incidence of subsequent symptomatic PDAs, and overall morb idity and mortality when compared with those with no evidence of PDA (silent or symptomatic). Prophylactic ductal closure decreased the incidence of subsequent PDA development, but had no effect on overall morbidity and/or mortality.
J Pediatr. 1978 Jul;93(1):110-3.
The silent ductus arteriosus.
McGrath RL, McGuinness GA, Way GL, Wolfe RR, Nora JJ, Simmons MA.
Preterm infants at risk of developing a patent ductus arteriosus were followed sequentially by physical examination, echocardiographic determinations of the LA/AO ratio, and chest roentgenograms. The results show that a significant number of infants who h ave no clinical signs or symptoms of a PDA have large left-to-right shunts. The presence of this shunt was suggested by acute increase in left atrial size by ECHO determination and confirmed by retrograde single-film aortography. Clinical signs and sympto ms often developed several days after documentation of the left-to-right shunt.
e-mail comments to: Dr. Hany Aziz
Natural History of PDA (Campbell, 1968)
Purpose: To attempt to describe the natural history and disease process of untreated PDA - from birth to late adulthood - especially pertaining to spontaneous closure and mortality rate.
Methods: Review of data on clinical course of patients with PDA over a 20 yr period from the early nineteen hundreds.
Discussion: Mortality in < 12 months is 30% (+/- 10%) and this rate drops exponentially over the first 2 yrs. Also pulmonary hypertension developed in 3 to 4% of this population. Less than 20 yrs: mortality is 0.5%/yr. Between 20 and 30, mortality is 1
.5%/yr. Between 30 and 40, mortality is 2.5%/yr. Mortality becomes 4%/yr in late adulthood. Spontaneous closure rate after the first year of life is 0.6%/yr (meaning that 20% will have closed by age 60). By age 30, 20% will have died. By age 45, 42% w
ill have died and 19% have spontaneously closed ductus. By age 60, 61% (+/- 10%) have died and spontaneously closed ductus in 21% (+/- 3%). Average age of death (excluding deaths in infancy) was 30-32yrs. 33-45% of PDA deaths related to SBE (0.45% annu
al risk) and 30% related to CHF. The remainder were related to other cardiovascular causes (7%) and non-cardiac and uncertain causes (18%). With the introduction of antibiotics for the prevention of endocarditis, mortality rate decreased in midlife but i
ncreased again later in life as there were more patients who developed CHF resulting in only a mild increase in the number of living at age 60. A higher incidence in females was noted (1:2.3). The authors recommend surgical closure (which provides norma
l life expectancy) for anyone without evidence of a closing ductus.
Provides a good idea of natural history (with respect to mortality vs spontaneous closure or development of CHF) of PDA during the advent of antibiotic therapy and surgical closure.
Auscultatorily Silent PDA (2 cases) (Abbott, 1973)
Case 1 is a 21 yr female presented with pulmonary embolus found to have silent PDA on catheterization; refused surgery and was placed on antibiotic prophylaxis for endocarditis.
Case 2 is a 10 yr girl who presented with syncopal attacks (diagnosed as vasovagal episodes) was found to have AS and AR as well as a "silent" PDA (masked PDA); she underwent surgical ligation.
Discussion: silent PDA not in the setting of elevated pulmonary pressures is rare. Natural history of PDA is unknown. It is speculated that there is a 10-30% of infective endocarditis with PDA. Surgical recommendations are made for 1) endocarditis pre
vention 2) hemodynamically significant PDA 3) hemodynamically insignificant PDA in children over 10 years of age. Surgery may be delayed for adults with hemodynamically insignificant ductus. Surgery is contraindicated if silent PDA is due to increased pu
lmonary pressures.
Aside from infective endocarditis, pulmonary embolus may be another complication of PDA that should warrant consideration for closure.
The Silent Ductus Arteriousus (McGrath,1978)
Purpose: To provide data on incidence and evolution of (silent) PDA with RDS, and response of treatment.
Result: 8/39 infants with severe respiratory distress syndrome were found to have significant silent PDA diagnosed by echo and retrograde aortography. All were treated (digitalis, furosemide, fluid restriction [not indomethacin]) Clinical signs and symp
toms developed after several days in all 8. Four of the 8 required surgical ligation due to lack of clinical improvement.
Discussion: silent PDA must be considered in any infant with severe respiratory distress. Earlier treatment of PDA (when silent) may improve medical management in premature infants with severe respiratory distress syndrome (reduced O2 exposure and barot
rauma).
Signs and symptoms of PDA developed 24-48 hrs after radiologic diagnosis despite treatment, possibly because indomethacin was not used at that time.
Letter to the Editor (Kashani, 1983)
Purpose: The writer takes issue with Ellison for underestimating the incidence of silent PDA in premature infants.
Discussion: Incidence of PDA/silent PDA is much higher than suggested with and without respiratory distress syndrome. The majority of premature infants with respiratory distress syndrome have a PDA. Left atrial to aorta ratio does not correlate well wi
th incidence of PDA. Radiographic findings (cardiomegaly and increased vasculature), hyperactive precordium, and CO2 retention and acidosis as more reliable signs. Absence of a murmur is attributed to low/no turbulence from a large PDA (nearly equal to
aortic diameter). Echos are obtained on all babies with suspected PDA to rule out other CVS anomalies like duct dependent lesions. Treatment is by fluid restriction and ventilatory support, if no improvement then indomethacin (surgery if indomethacin is
contraindicated).
Left atrial to aorta ratio was used in McGrath's 1978 paper to diagnose silent PDA: incidence was 8/39 of severe respiratory distress syndrome pts (20%). Using this method (assuming Kashani is correct), McGrath underestimated the true incidence of silent
PDA.
The Silent Ductus: its Precursors and its Aftermath (Hammerman, 1986)
Purpose: 1) To determine potential risk factors involved in the development of the silent ductus, 2) its impact upon both the early cardiorespiratory symptomatology and the subsequent morbidity of the premature neonate, and finally 3) the potential benef
it to be derived from prophylactic closure in this presymptomatic stage.
Methods: 31 infants <1kg screened clinically and by echo for PDA and divided into 3 groups randomly (no signs of PDA, silent PDA untreated, and silent PDA treated prophylactically. The 3 groups were observed for incidence of morbidities and mortality.
Results: Infants with PDA had increased levels of PGE. Infants with silent PDA had increased incidence of development of significant PDA. However, prophylactic duct closure had no effect on overall morbidity or mortality.
Discussion: Incidence of PDA in < 1kg is 60% as previously reported. Manifestations of respiratory distress syndrome felt largely due to L-R shunt and to a lesser extent from surfactant deficiency. There may be some unidentified risk factor that leads
to morbidity and mortality associated with PDA.
Where infants > 1kg already studied under similar circumstances in a previous study: 79% of PDA's that were treated, closed. Effect of different treatment (5 day course vs the 3 dose regimen used [if closure rate was low]). Echocardiographic criteria we
re used to diagnose PDA: (if LA-Ao ratio, this is not as sensitive as Doppler echo; furthermore, in contrast echos... are false positives (ASD's) falsely included in the study?) Early treatment with surfactant with very early treatment with indomethacin
may be effective.
Doppler Ultrasound and the Silent PDA (Houston, 1991)
Purpose: To present the case that incidence of PDA is higher than previously shown by less sensitive diagnostic methods (as compared with Doppler ultrasound). To speculate that endocarditis patients with no evident underlying structural heart disease ma
y have a silent PDA.
Methods: 21 patients older than 12 months diagnosed with silent PDA by Doppler ultrasound in a population of 7,345 children. Most were referred for evaluation of murmurs.
Discussion: silent PDA due to large duct, coincident pulmonary hypertension, or trivial duct. This study dealt with the last category - which would not have been diagnosed without Doppler ultrasound.
The authors conclude that 0.5% of those presenting with innocent murmurs have a silent PDA. It is difficult to estimate the incidence in the general population, but it is likely to be higher than the previously published 0.065% incidence. The practical i
mplication is for appropriate endocarditis prophylaxis.
Kashani's estimate (in 1983) may be still lower than the actual incidence, as this was a clinical rather than Doppler diagnosis.
Letters to the Editor (1993) 4 commentators:
1) Sturridge: Does not support treatment of asymptomatic PDA; calls for a national study to evaluate morbidity and mortality of untreated small shunts.
2) Glickstein: States that small PDA is distinguished from silent PDA by presence of reversal of diastolic flow in the thoracic aorta. Theorizes that there is no murmur because of no turbulence and therefore no endothelial damage and therefore no recomm
endation for antibiotic prophylaxis for endocarditis.
3) Balzer: Presents a [first] case of SBE in a silent PDA of a 19yr old male. And offers the recommendation that all PDA's should be considered for closure and antibiotic prophylaxis.
4) Lloyd: Refutes Balzer's implication that all children should be screened and treated for silent PDA. Risks and costs heavily outweigh the benefits. Estimates incidence of silent PDA to be 0.5% of all children; and 1% of children studied by echo for
Kawasaki's disease. Does not recommend surgery or prophylactic antibiotics for silent PDA.
Transcatheter Occlusion of PDA: Which Method to Use and Which Ductus to Close (Syamuasundar, 1996)
Purpose: To review different devices, techniques, complications, and indications for ductal closure.
Methods: Literature review of various clinical trials and animal trials.
Result: Recommends closure of PDA <3.5mm with coils, and PDA >3.5mm with adjustable button device (least complications and highest success rates). Concurs with prevalence of PDA to be 0.5 to 1% of patients undergoing echo for unrelated reasons and advis
es closure for any PDA with continuous murmur with echo Doppler confirmation (same indication as for surgery).
Many PDA's don't have a continuous murmur, but only a "partial" systolic murmur - authors didn't comment on this group. Concur with previous writers that incidence of endarteritis is too low to recommend closure.
Back to E-chocardiography Home Page.
e-mail:shindler@umdnj.edu
The contents and links on this page were last verified on January 24, 2005.