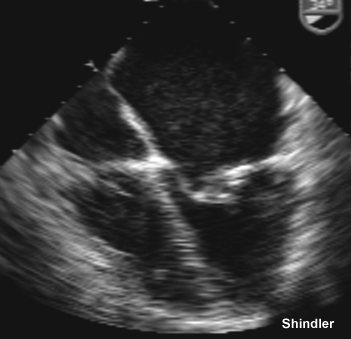
 Mitral stenosis.
Mitral stenosis.
 Mitral stenosis.
Mitral stenosis.
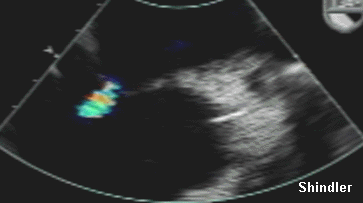 Left to right shunt across a distended
atrial septum in the same patient.
Left to right shunt across a distended
atrial septum in the same patient.
Ananthasubramaniam K, Iyer G, Karthikeyan V. Giant left atrium secondary to tight mitral stenosis leading to acquired Lutembacher syndrome: a case report with emphasis on role of echocardiography in assessment of Lutembacher syndrome. J Am Soc Echocardiogr 2001 Oct;14(10):1033-5
Lutembacher syndrome is an unusual clinical entity of congenital secundum atrial septal defect in combination with rheumatic mitral stenosis. Although this classic form is seldom seen by the adult cardiologist, spontaneous Lutembacher syndrome as discussed later or the iatrogenic variant is not infrequently encountered. The pathophysiologic, clinical, and hemodynamic differences of mitral valve disease in the presence of atrial septal defect compared with isolated mitral stenosis are highlighted in this case review. Special emphasis has also been given to echocardiographic evaluation of this syndrome complex, particularly in the setting of percutaneous mitral valvuloplasty, which produces the iatrogenic form of Lutembacher syndrome.
Vasan RS, Shrivastava S, Kumar MV. Value and limitations of Doppler echocardiographic determination of mitral valve area in Lutembacher syndrome. J Am Coll Cardiol 1992 Nov 15;20(6):1362-70
OBJECTIVES. Our objective was to compare the Doppler pressure half-time, Doppler continuity equation and two-dimensional echocardiographic planimetric methods of estimating mitral valve area in Lutembacher syndrome. BACKGROUND. Fluid dynamics theory predicts that mitral pressure half-time varies inversely with mitral valve area and directly with net chamber compliance and the peak early diastolic transmitral gradient in pure mitral stenosis. The effects of an atrial shunt on these interrelations have not been investigated. METHODS. Correlation and agreement between mitral valve area estimates obtained by the three methods and that obtained by cardiac catheterization was ascertained in 11 patients with Lutembacher syndrome. RESULTS. Valve areas determined by planimetry and the continuity equation method correlated and agreed well with catheterization measurements (r = 0.83 and 0.81, respectively). The pressure half-time method consistently overestimated mitral valve area; the extent of overestimation was greater in patients with larger atrial shunts. The hemodynamic pressure half-time was independent of the mitral valve area, chamber compliance and the peak transmitral gradient. It was dependent on the magnitude of the atrial shunt, although the correlation obtained was only fair (r = 0.61). CONCLUSIONS. These findings suggest that the Doppler pressure half-time method is an inaccurate measure of mitral valve area whenever an atrial shunt coexists with mitral stenosis. Planimetry and the Doppler continuity equation methods yield accurate estimates of mitral valve area in Lutembacher syndrome.
Fadel BM, Hiatt BL, Kerins DM. Isolated Rheumatic Tricuspid Stenosis with Reverse Lutembacher's Physiology. Echocardiography 1999 Aug;16(6):567-573
Tricuspid valve dysfunction occurs frequently in patients with rheumatic heart disease and is usually manifested as functional or organic tricuspid regurgitation. Rheumatic tricuspid stenosis is less common and occurs characteristically in the presence of concomitant mitral valve disease. In this report, we describe the clinical and echocardiographic findings in a patient with isolated rheumatic tricuspid stenosis and a right-to-left shunt across the interatrial septum, likely as a result of a patent foramen ovale, resulting in central cyanosis. This case illustrates an interesting association of tricuspid stenosis and an interatrial right-to-left shunt suggestive of a reverse Lutembacher's physiology.
Cheng TO. Coexistent atrial septal defect and mitral stenosis (Lutembacher syndrome): An ideal combination for percutaneous treatment. Catheter Cardiovasc Interv 1999 Oct;48(2):205-6 Comment on: Catheter Cardiovasc Interv. 1999 Oct;48(2):199-204.
Joseph G, Abhaichand Rajpal K, Kumar KP. Definitive percutaneous treatment of Lutembacher's syndrome. Catheter Cardiovasc Interv 1999 Oct;48(2):199-204
Definitive percutaneous treatment of a patient with Lutembacher's syndrome was successfully accomplished using the Amplatzer septal occluder to close a secundum atrial septal defect and the Joseph mitral balloon catheter to dilate rheumatic mitral valve stenosis. Transcatheter therapy is an effective alternative to surgery in selected patients with Lutembacher's syndrome.
Megarbane A, Stephan E, Kassab R, Ashoush R, Salem N, Bouvagnet P, Loiselet J. Autosomal dominant secundum atrial septal defect with various cardiac and noncardiac defects: a new midline disorder. Am J Med Genet 1999 Mar 19;83(3):193-200
We report on a Lebanese family in which 12 persons had an atrial septal defect and various cardiac and noncardiac anomalies. Cardiac anomalies are left axis deviation of QRS, right bundle branch block, atrial fibrillation, Wolff-Parkinson-White syndrome, nodal atrioventricular rhythm, aortic stenosis, pulmonic valve stenosis, mitral stenosis (Lutembacher syndrome), and low implantation of the tricuspid valve (Ebstein disease). Noncardiac abnormalities consisted specially of the presence of hypertelorism, cleft lip, and pectus excavatum. This combination appears to constitute a hitherto undescribed autosomal dominant midline disorder of the heart and upper half of the body with almost full penetrance and variable expressivity. The mutation does not map to any known locus involved in atrial septal defect or conduction block.
Wiedemann HR. Earliest description by Johann Friedrich Meckel, Senior (1750) of what is known today as Lutembacher syndrome (1916). Am J Med Genet 1994 Oct 15;53(1):59-64
The letter that the famous anatomist Johann Friedrich Meckel, Sr. sent from Berlin on May 5, 1750 to the great Albrecht von Haller (at that time resident in Gottingen) contains the earliest reference to an unusual observation made by the former. Even today this observation is considered in the clinical literature to be the first description of a coarctation of the aorta. In fact, it is probably the first description of what is known today as Lutembacher syndrome.
Iga K, Tomonaga G, Hori K. Continuous murmur in Lutembacher syndrome analyzed by Doppler echocardiography. Chest 1992 Feb;101(2):565-6
A continuous murmur was heard in a 47-year-old woman with Lutembacher syndrome. Transesophageal and intraoperative Doppler echocardiography revealed the murmur originating from the accelerated blood flow passing through the small atrial septal defect. To our knowledge, this is the first reported case of a continuous murmur in Lutembacher syndrome analyzed by Doppler echocardiography.
Kimura M, Kitasato K, Kamatani M, Fujino T. Long-term follow-up of cardiac rhythms after biatrial transseptal approach (Dubost's incision). Fukuoka Igaku Zasshi 1992 Feb;83(2):57-61
Biatrial transseptal approach (Dubost's incision) was performed 54 times on 47 patients from November 1973 to December 1982 at National Fukuoka Higashi Hospital in Japan. The patients consisted of 19 males and 28 females, with ages ranging from 14 to 66 (mean 45.9 years). Forty-four out of 47 cases had rheumatic heart disease while one had endocardial cushion defect, one had Lutembacher's syndrome, and one had left atrial myxoma. Preoperative electrocardiograph showed atrial fibrillation in 37 cases (78.7%) and normal sinus rhythm in 10 cases (21.3%). The follow-ups of the patients were a minimum of 8 years and a maximum of 16 years (mean 9.6 years) with 97.8% completion. Cumulative follow-up period was 448.9 patient years. Postoperatively, atrial fibrillation persisted in all except two. In those patients, normal sinus rhythm was observed until postoperative six months and seven years, respectively. Normal sinus rhythm persisted in six cases, and changed into junctional rhythm in four. One of them changed into atrial fibrillation at 10.6 years postoperatively. We conclude that Dubost's incision provides an excellent operative field for mitral and tricuspid valve surgery without serious internodal conduction disturbances.
Sheikhzadeh A, Moghbeli H, Ghabussi P, Tarbiat S. Severe tricuspid valve stenosis. A cause of silent mitral stenosis. Jpn Heart J 1983 Jul;24(4):563-70
The diastolic rumbling murmur of mitral stenosis (MS) may be attenuated in the presence of low cardiac output, right ventricular enlargement, Lutembacher's syndrome, pulmonary emphysema, and obesity. In this report we would like to stress that the presence of tricuspid stenosis (TS) is an additional significant cause of silent MS. The clinical material consisted of 73 patients with rheumatic TS who had undergone cardiac surgery. Five of these cases had clinical findings of TS without auscultatory findings of MS. They were found to have severe MS at the time of operation and to require mitral valve surgery. At cardiac catheterization the mean diastolic gradient (MDG) across the mitral valve (MV) was less than 3 mmHg and pulmonary arterial systolic pressure was 29-42 mmHg. The MDG across the tricuspid valve was 6-17 mmHg. In conclusion, TS can mask clinical and hemodynamic findings of MS. The reason for this is the mechanical barrier imposed by TS proximal to the MV.
Back to E-chocardiography Home Page.

The contents and links on this page were last verified on October 31, 2006.