
Frequent regurgitation of acidic fluids from the stomach into the lower esophagus is clinically termed as Gastroesophageal reflux. Symptoms of such reflux include heartburn and chest pain. Estimated 18.1% to 27.8% in the USA and 8.8% to 25.9% in Europe suffer from gastroesophageal reflux disease (GERD) 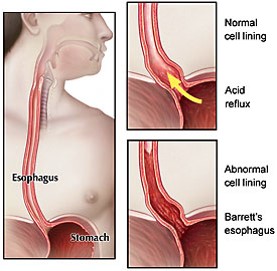
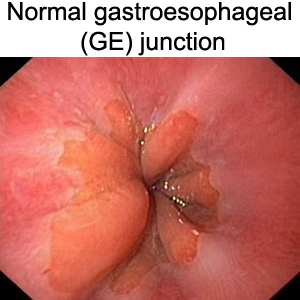
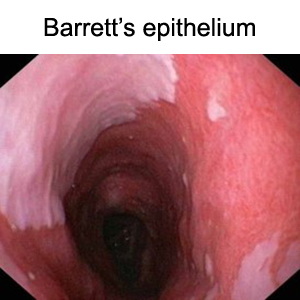
Barrett’s metaplasia or Barrett’s epithelium (BE) is a specialized tissue that develops at the junction of the esophagus (food pipe) and stomach mostly in patients with gastroesophageal reflux disease (GERD). About 5.6 percent of adults in the United States have Barrett's esophagus 2. Patients with BE are clinically predisposed to a 120-fold higher risk of developing EA. BE may advance into esophageal adenocarcinoma (EA) following the metaplasia→dysplasia→cancer sequence. Esophageal adenocarcinoma is a fatal cancer with less than 15%, 5 year survival and has been on the rise in the US and western world.
Dr. Bajpai’s research team has made seminal efforts toward the development and characterization of the disease models to study origin and pathogenesis of Barrett’s metaplasia and esophageal adenocarcinoma. The current and future investigations are focused toward identification of causative dysregulated biological processes, therapeutic approaches and early diagnosis of Barrett’s esophageal adenocarcinoma.
1. Dynamic "in-vitro" disease models to study origin and pathogenesis of Barrett’s epithelium.
a. gastroesophageal reflux induces reprogramming or trans-commitment of squamous epithelial cells leading to columnar Barrett’s metaplasia
references: https://www.ncbi.nlm.nih.gov/pubmed/28336546 ![]()
b. chronic gastroesophageal reflux induces progression of Barrett’s epithelial metaplasia to esophageal adenocarcinoma.
references: https://www.ncbi.nlm.nih.gov/pubmed/18427553 ![]()
https://www.ncbi.nlm.nih.gov/pubmed/20309934 ![]()
c. Chronic gastroesophageal reflux induces genetic and epigenetic changes in the Barrett’s epithelial metaplasia to esophageal adenocarcinoma.
references: https://www.ncbi.nlm.nih.gov/pubmed/24123713 ![]()
https://www.ncbi.nlm.nih.gov/pubmed/23194200 ![]()
These cell line-derived disease models developed in our laboratory are currently the most tractable, reproducible and affordable tools for Barrett’s pathogenesis research.
Work in progress:
Investigating genetic and epigenetic mechanisms of pathogenesis and therapeutic approaches to prevent development of Barrett’s metaplasia and Esophageal adenocarcinoma

Biomarker discovery using genomics methods:
Integration of HiSeq Illumina based RNA sequencing for gene expression and mutational status. HELP Tagging Assay for DNA methylation status and subsequent computational analyses using Ingenuity pathway, Gene Ontology, and KEGG PATHWAY analyses revealed striking genetic and epigenetic changes in the esophageal epithelial cells after chronic acid and bile exposure. These changes resulted in altered cellular differentiation with disrupted cellular functions that promote carcinogenesis.
references: https://www.ncbi.nlm.nih.gov/pubmed/24123713 ![]()
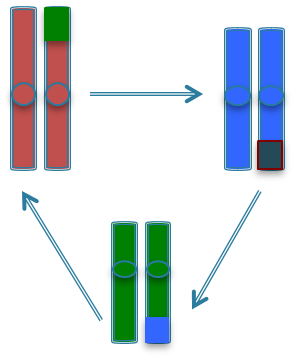
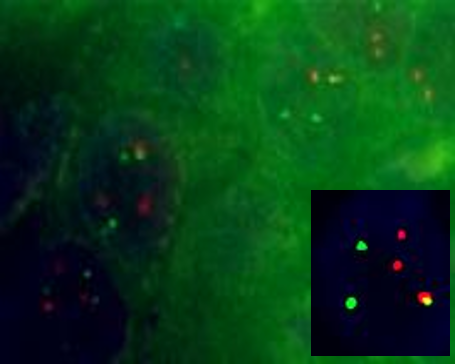
Biomarker discovery and validation using conventional fluorescent in-vitro hybridization FISH
Dr. Bajpai lead investigations that discovered several structural changes in the chromosomes that appear before malignant transformation of the BE cells in a human gastroesophageal reflux disease model. In pilot studies, these chromosomal events were detected in human EA with the help of novel DNA probes and fluorescence in-situ hybridization (FISH) assay and not in the benign BE tissues, These events therefore may enable screening patients with Barrett’s epithelium for the risk of developing esophageal adenocarcinoma and personalized therapy for better prognosis.
references: https://www.ncbi.nlm.nih.gov/pubmed/23194200 ![]()
Patent application
U.S. Patent Application No. PCT/2017/021405
Inventors: Manisha Bajpai, Hana Aviv and Kiron M. Das read more
Work in progress
Chemoprevention aims to prevent and or delay the process of cancer development in individuals with premalignant conditions or genetic predisposition. Several pharmaceutical drugs (synthetic chemicals) and nutraceuticals (natural nutrients derived from food) are being evaluated for their ability to effectively delay or prevent cancer development without severe side-effects to the otherwise healthy individuals.
Work in progress
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) of unknown etiology and pathogenesis and is a strong risk factor for development of colorectal cancer (CRC). Currently, about 1-1.3 million people in USA live with IBD.
a. Autoimmunity to human tropomyosins in ulcerative colitis
Autoimmunity is an important pathogenic process in UC marked by the presence of autoantibodies in UC against human tropomyosin isoform 5 (hTM5). Tropomyosins (TMs) are cytoskeletal microfilament-associated proteins present in all eukaryotic cells. Dr. Bajpai participated in the investigations conducted by Drs. that demonstrated that elevated levels of anti-hTM5 IgG autoantibodies in the UC patients serum participated in glandular destruction, a hallmark of ulcerative colitis.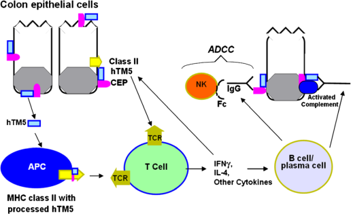
references: https://www.ncbi.nlm.nih.gov/pubmed/19690525 ![]()
https://www.ncbi.nlm.nih.gov/pubmed/19076830 ![]()
b. Molecular Biomarkers of Disease Progression in Ulcerative Colitis and Colorectal Cancer
TC-22 an isoform of hTM5 is a “biomarker”, previously discovered in the Das laboratory. It is only found in the UC and colorectal cancer patients and not in the colonic mucosa of normal subjects. Dr. Bajpai’s studies established that 5-ASA or mesalamine, a drug commonly used in treatment of UC patients actually inhibits the expression of TC22 in colorectal cancer.
references: https://www.ncbi.nlm.nih.gov/pubmed/19369484 ![]()
Work in progress